how many valence electrons does hydrogen have|Determine valence electrons using the periodic table : Clark Find out the valences of the chemical elements, including hydrogen, from a . Discover the excitement at Clashodds! Register now and claim your P999 free bonus. Hurry, join today for thrilling online gaming experiences! . From classic casino games like blackjack and roulette to modern slots and live dealer games, the platform offers a diverse selection. Slot enthusiasts will find a variety of options, from traditional .The winners of the STARS lottery grand prizes were announced on Tuesday. Mike O’Keefe from Saskatoon is the winner of the grand prize show home in Regina. The home on Green Stone Road is valued .
PH0 · Valences of the Chemical Elements
PH1 · Valence electrons and ionic compounds (video)
PH2 · Valence electrons (video)
PH3 · Valence electron
PH4 · Valence Electrons Chart for All Elements
PH5 · How to Find the Valence Electrons for Hydrogen (H)?
PH6 · How many valence electrons does hydrogen have?
PH7 · How Many Valence Electrons Does Hydrogen (H)
PH8 · Determine valence electrons using the periodic table
PH9 · 3.10: Valence Electrons
PH10 · 10.6: Valence Electrons
Anúncios de escorts e garotas de programa coroas para ter sexo com acompanhantesem Campinas. Eliminados 200.710 anúncios com fotos falsas. Categoria: Acompanhantes Massagens Videochamadas. . Mostrar mais acompanhantes. errorNão encontramos anúncios que cumpram os critérios de busca. expand_less. Conteúdo somente para .
how many valence electrons does hydrogen have*******Mar 23, 2023 how many valence electrons does hydrogen have Determine valence electrons using the periodic table Learn how to determine the number of valence electrons for an element using the periodic table. Hydrogen has one valence electron in group 1, while helium has t. Find out the valences of the chemical elements, including hydrogen, from a .
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as . Learn how to calculate the valence electrons of hydrogen (H) by following three steps: determining the total number of electrons, arranging them in shells, and identifying the valence shell. Also, find .
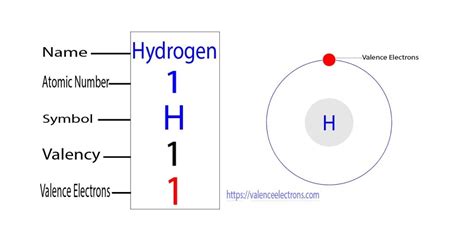
Each hydrogen atom has one valence electron and is univalent. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can . Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; .
Hydrogen can exist without proton if it is in its ionised form - i.e. as H+. H+ is just a proton, so no electrons would be present. In covalent bonding, the electrons that form the covalent bond do not have to come from each atom. There are instances .
Determine valence electrons using the periodic table Hydrogen has 1 valence electron. Explanation: Hydrogen is in the first row of the Periodic Table. Elements in the first row are filling their 1s orbitals. Since hydrogen is the first element, its electron .

The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated .The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated .
sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.
This table of element valences includes the maximum valence and most common valence values in chemistry. . (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Remember an element's electron cloud will become more stable by filling, emptying, or half-filling the .
There are two ways to find the number of valence electrons in Hydrogen (H). The first is to use the Periodic Table to figure out how many electrons Hydrogen .
The electron configuration of a hydrogen atom is spoken out loud as “one-ess-one.” . How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels and so the answer is three. In fact, the number of valence electrons goes up by one for each step .
how many valence electrons does hydrogen have Beryllium has two valence electrons. How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period, until the last element is . Valence shell electrons (or, more simply, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. We can see from the electron configuration of a carbon atom—1 s2 2 s2 2 p2 —that it has 4 valence electrons (2 s2 2 p2) and 2 core .How many Valence Electrons does Hydrogen have? Q: How many Valence Electrons does Hydrogen have? How many Valence Electrons does Hydrogen have? Flexi Says: It only has one valence electron. Discuss further with Flexi. Ask your own question! Want to learn more? Go to Lesson Page. ABOUT Our Mission; Meet the Team; Partners; .
When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most .Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .It has the lowest density of all gases. Uses. Some see hydrogen gas as the clean fuel of the future – generated from water and returning to water when it is oxidised. Hydrogen-powered fuel cells are increasingly being seen as ‘pollution-free’ sources of energy and are now being used in some buses and cars.
The valence electrons are the electrons in the outermost electron shell of an atom. That is why elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table. Generally, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell, corresponding to the electron configuration s2p6.
The number of valence electrons for each main group element can be determined by the column, or group, it occupies on the periodic table. Table 2.6.2 below summarizes the number of valence electrons for each main group column of elements. For example, the elements in the first column (sometimes labeled IA), all have one valence . Hence one hydrogen atom can form bonds with electrons of many atoms simultaneously. For example, four Hydrogen atoms can form a bond with a carbon atom, to form the compound methane (CH 4). Hydrogen Valence Electrons. Since Hydrogen belongs to the first group of elements its electronic configuration is 1. To determine the valence electrons of NH 3, it is first necessary to know the valence electrons of the hydrogen and nitrogen atoms. To determine the valence electrons of ammonia we have to follow two steps. It is shown below: Step 1: Determine the valence electrons of nitrogen and hydrogen atoms. The atomic number of nitrogen . To determine the valence electrons of C 2 H 6 O, it is first necessary to know the valence electrons of the carbon, hydrogen, and oxygen atoms. To determine the valence electrons of ethanol we have to follow two steps. It is shown below: Step 1: Determine the valence electrons of carbon, hydrogen, and oxygen atoms. The atomic .The valence (or valency) of an element is a measure of its combining power with other atoms when it forms chemical compounds or molecules. The concept of valence was developed in the last half of the 19th century and was successful in explaining the molecular structure of many organic compounds. The quest for the underlying causes of valence .
DepEd’s Eastern Visayas regional office had the biggest chunk of the unpaid Pag-Ibig deductions at P40.2 million, followed by the Cagayan Valley and the Bicol regional offices at P26.7 million .
how many valence electrons does hydrogen have|Determine valence electrons using the periodic table